MSymond1 Week 10
Contents
Purpose
The purpose of this lab procedure was to continue the transcriptomic analysis phase of the class by using the GO clusters and and terms, and using GRN modeling and visualization to help understand the transcription factors involved in cold shock for yeast cells.
Methods & Results
- I Prepared my microarray data file for loading into STEM.
- I inserted a new worksheet into your Excel workbook, and named it "(WT)_stem".
- I selected all of the data from the "(STRAIN)_ANOVA" worksheet and Paste special > paste values into your "(WT)_stem" worksheet.
- My leftmost column should have the column header "Master_Index". Rename this column to "SPOT". Column B should be named "ID". I Renamed this column to "Gene Symbol". Delete the column named "Standard_Name".
- I Filtered the data on the B-H corrected p value to be > 0.05 (that's greater than in this case).
- Once the data had been filtered, I selected all of the rows (except for your header row) and deleted the rows by right-clicking and choosing "Delete Row" from the context menu. I Undid the filter. This ensured that it clustered only the genes with a "significant" change in expression and not the noise"
- I Deleted all of the data columns EXCEPT for the Average Log Fold change columns for each timepoint.
- I Renamed the data columns with just the time and units (for example, 15m, 30m, etc.).
- I saved my work. Then used Save As to save this spreadsheet as Text (Tab-delimited) (*.txt). I clicked OK to the warnings and close your file.
- Note that you should turn on the file extensions if you have not already done so.
- I downloaded and extracted the STEM software. Click here to go to the STEM web site.
- I clicked on the download link and downloaded the
stem.zipfile to your Desktop. - I extracted the file by right-clicking on it and selecting "Extract all" from the menu.
- This created a folder called
stem.- I downloaded the Gene Ontology and yeast GO annotations and place them in this folder.
- I Clicked here to download the file "gene_ontology.obo".
- I Clicked here to download the file "gene_association.sgd.gz".
- Inside the folder, double-click on the
stem.jarto launch the STEM program.
- I clicked on the download link and downloaded the
- Running STEM
- In section 1 (Expression Data Info) of the the main STEM interface window, I clicked on the Browse... button to navigate to and select your file.
- I clicked on the radio button No normalization/add 0.
- I checked the box next to Spot IDs included in the data file.
- In section 2 (Gene Info) of the main STEM interface window, I left the default selection for the three drop-down menu selections for Gene Annotation Source, Cross Reference Source, and Gene Location Source as "User provided".
- I clicked the "Browse..." button to the right of the "Gene Annotation File" item. I Browsed to your "stem" folder and select the file "gene_association.sgd.gz" and click Open.
- In section 3 (Options) of the main STEM interface window, I made sure that the Clustering Method says "STEM Clustering Method" and do not change the defaults for Maximum Number of Model Profiles or Maximum Unit Change in Model Profiles between Time Points.
- In section 4 (Execute) I clicked on the yellow Execute button to run STEM.
- A new window opened called "All STEM Profiles (1)". Each box corresponded to a model expression profile. Colored profiles had a statistically significant number of genes assigned; they were arranged in order from most to least significant p value. Profiles with the same color belong to the same cluster of profiles. The number in each box is simply an ID number for the profile.
- I clicked on the button that says "Interface Options...". At the bottom of the Interface Options window that appeared below where it says "X-axis scale should be:", I clicked on the radio button that says "Based on real time". Then closed the Interface Options window.
- I took a screenshot of this window (on a PC, simultaneously press the
AltandPrintScreenbuttons to save the view in the active window to the clipboard) and paste it into a PowerPoint presentation to save your figures.
- I clicked on each of the SIGNIFICANT profiles (the colored ones) to open a window showing a more detailed plot containing all of the genes in that profile.
- I took a screenshot of each of the individual profile windows and saved the images in your PowerPoint presentation.
- At the bottom of each profile window, there were two yellow buttons "Profile Gene Table" and "Profile GO Table". For each of the profiles, I clicked on the "Profile Gene Table" button to see the list of genes belonging to the profile. In the window that appeared, I clicked on the "Save Table" button and save the file to your desktop. I made the filename descriptive of the contents, e.g. "wt_profile#_genelist.txt", where I replaced the number symbol with the actual profile number.
- I uploaded these files to the wiki and linked to them on your individual journal page. Note that it was easier to zip all the files together and upload them as one file. To do this, I selected all of the files you want to zip together. Then right clicked and selected "Send to" and "Compressed (zipped) folder" from the context menu.
- For each of the significant profiles, I clicked on the "Profile GO Table" to see the list of Gene Ontology terms belonging to the profile. In the window that appears, I clicked on the "Save Table" button and save the file to your desktop. Make your filename descriptive of the contents, e.g. "wt_profile#_GOlist.txt", where you use "wt", "dGLN3", etc. to indicate the dataset and where you replace the number symbol with the actual profile number. At this point you have saved all of the primary data from the STEM software and it's time to interpret the results!
- I uploaded these files to the wiki and linked to them on the individual journal page. Note that it was be easier to zip all the files together and upload them as one file. To do this, I selected all of the files I wanted to zip together. Then right clicked and selected "Send to" and "Compressed (zipped) folder" from the context menu.
- In section 1 (Expression Data Info) of the the main STEM interface window, I clicked on the Browse... button to navigate to and select your file.
- Analyzing and Interpreting STEM Results
- I selected profile 9 which I saved in the previous step for further intepretation of the data. It is suggested to choose one that has a pattern of up- or down-regulated genes at the cold shock timepoints. Each member of your group should choose a different profile. Answer the following:
- I chose profile 9 because it has very dynamic lines and a wide range of values across all of the lines.
- There are 200 genes assigned to this profile
- There are 60.3 genes expected to belong to this profile
- The p-value for this profile is 3.1E-48
- I opened the GO list file I saved for this profile in Excel. This list showed all of the Gene Ontology terms that are associated with genes that fit this profile. I Selected the third row and then chose from the menu Data > Filter > Autofilter. I filtered on the "p-value" column to show only GO terms that have a p value of < 0.05. 28 GO terms are associated with a p-value of less than .05 The GO list also had a column called "Corrected p-value". This correction was needed because the software had performed thousands of significance tests. I filtered on the "Corrected p-value" column to show only GO terms that have a corrected p value of < 0.05. 4 GO terms are associated with a corrected p-value of less than .05
- I selected 6 Gene Ontology terms from your filtered list (either p < 0.05 or corrected p < 0.05).
- Each member of the group reported on his or her own cluster in your research presentation. I chose terms that are the most significant, but that are also not too redundant. For example, "RNA metabolism" and "RNA biosynthesis" are redundant with each other because they mean almost the same thing.
- I noted whether the same GO terms are showing up in multiple clusters.
- I Looked up the definitions for each of the terms at http://geneontology.org.
- To easily look up the definitions, I went to http://geneontology.org.
- I copied and pasted the GO ID (e.g. GO:0044848) into the search field on the left of the page.
- In the results page, I clicked on the button that says "Link to detailed information about <term>, in this case "biological phase"".
- The definition was on the next results page, e.g. here.
- vesicle-mediated transport (GO:0016192): A cellular transport process in which transported substances are moved in membrane-bounded vesicles; transported substances are enclosed in the vesicle lumen or located in the vesicle membrane. The process begins with a step that directs a substance to the forming vesicle, and includes vesicle budding and coating. Vesicles are then targeted to, and fuse with, an acceptor membrane.
- Golgi apparatus (GO:0005794): A membrane-bound cytoplasmic organelle of the endomembrane system that further processes the core oligosaccharides (e.g. N-glycans) added to proteins in the endoplasmic reticulum and packages them into membrane-bound vesicles. The Golgi apparatus operates at the intersection of the secretory, lysosomal, and endocytic pathways.
- cytoplasmic translation (GO:0002181): The chemical reactions and pathways resulting in the formation of a protein in the cytoplasm. This is a ribosome-mediated process in which the information in messenger RNA (mRNA) is used to specify the sequence of amino acids in the protein.
- protein transport (GO:0015031): The directed movement of proteins into, out of or within a cell, or between cells, by means of some agent such as a transporter or pore.
- ribosomal small subunit assembly (GO:0000028): The aggregation, arrangement and bonding together of constituent RNAs and proteins to form the small ribosomal subunit.
- transaminase activity (GO:0008483): Combining with the neurotransmitter dopamine and activating adenylate cyclase via coupling to Gi/Go to initiate a change in cell activity.
- The cells react to cold shock by changing expression of genes associated with these terms because these terms because they all relate to protein synthesis/transport and enzymatic activities, which are slowed down by cold shock in yeast cells.
- Each member of the group reported on his or her own cluster in your research presentation. I chose terms that are the most significant, but that are also not too redundant. For example, "RNA metabolism" and "RNA biosynthesis" are redundant with each other because they mean almost the same thing.
- I selected profile 9 which I saved in the previous step for further intepretation of the data. It is suggested to choose one that has a pattern of up- or down-regulated genes at the cold shock timepoints. Each member of your group should choose a different profile. Answer the following:
Using YEASTRACT to Infer which Transcription Factors Regulate a Cluster of Genes
- I opened the gene list in Excel for the one of the significant profiles from the stem analysis that you chose to perform the GO analysis. It was a cluster with a clear cold shock/recovery up/down or down/up pattern, and was one of the largest clusters.
- I Copied the list of gene IDs onto the clipboard.
- I launched a web browser and go to the YEASTRACT database.
- On the left panel of the window, I clicked on the link to Rank by TF.
- I pasted the list of genes from your cluster into the box labeled ORFs/Genes.
- I checked the box for Check for all TFs.
- I accepted the defaults for the Regulations Filter (Documented, DNA binding or expression evidence)
- I Did not apply a filter for "Filter Documented Regulations by environmental condition".
- I ranked genes by TF using: The % of genes in the list and in YEASTRACT regulated by each TF.
- I clicked the Search button.
- In the results window that appears, the p values colored green are considered "significant", the ones colored yellow are considered "borderline significant" and the ones colored pink are considered "not significant". 25 of the genes were green and significant
- I Copied the table of results from the web page and paste it into a new Excel workbook to preserve the results.
- I Copied by selecting and dragging down on the table.
- When pasting into Excel, I remembered to Paste special > Paste values.
- I Uploaded the Excel file to the wiki and linked to it in my electronic lab notebook.
- CIN5 is in the table, 39.00% is in user set, 3.39% is in scerevisiae, and the p-value is 0.005141327836732. GLN3 is also in the database, 34.00% is in the user set, 2.76% is in scerevisiae, and the p-value is 0.346793143826899.
Creating and Visualizing Your Gene Regulatory Network with GRNsight (Tuesday, March 26)
- I selected from the list of "significant" transcription factors in YEASTRACT, which ones you will use to run the model. I used these transcription factors and added GLN3 and CIN5.
- Generally, I included the top transcription factors with the smallest p values. Each group member selected a different network.
- I went to the GRNsight beta website.
- Under the "Network" panel on the left-hand side, I clicked the button "Load from database".
- I typed the standard name of the transcription factor in the "Select gene" field and clicked the find button (magnifying glass).
- I continued to add transcription factors in this way until I had 15-20.
- I clicked the "Generate Network" button.
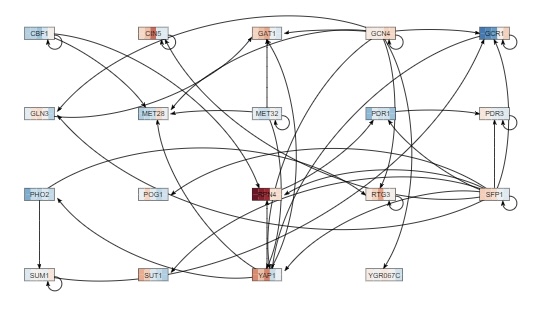
- my network appeared on the screen.
- I checked to see if all of the rectangular boxes (nodes) are connected by at least one arrow to another node. If there was not a node that is connected, I went back to the "Load from database" button and selected the transcription factors again, leaving out the node that was disconnected.
- My network had 19 genes and 41 edges.
- Under the "Layout" section, clicked on the "Grid Layout" button.
- I exported the network image by going to the Export menu and selecting "Export Image > To PNG". The picture is to the right
Creating the GRNmap Input Workbook
We will also use GRNsight to automatically generate the input workbook for the GRNmap modeling software. Note that this feature is still under development, and we will be performing quality control on the exported workbook.
- With your final network still open in GRNsight, I selected from the Export menu "Export Data > To Excel". In the window that appears, I selected the following:
- Under "Select the Expression Data Source:", I chose "Dahlquist_2018"
- Under "Select Workbook Sheets to Export:", I selected the following:
- Network sheets
- "network"
- Expression sheets
- dcin5_log2_expression
- dgln3_log2_expression
- wt_log2_expression
- Additional sheets
- "degradation_rates"
- "optimization_parameters"
- "production_rates"
- "threshold_b"
- Network sheets
- I clicked the "Export Workbook" button.
- I opened the workbook in Excel to perform quality control. I checked that it has the following sheets with the following content:
- The "network" sheet had an adjacency matrix with your selected regulatory transcription factors across the top row and in the first column.
- The "dcin5_log2_expression", "dgln3_log2_expression", and "wt_log2_expression" sheets had log2 fold changes for each of your selected regulatory transcription factors for each time point (15, 30, 60, 90, 120). Replicate values have the same column headers. If a particular gene is missing all 4-5 replicate values at a particular timepoint for a particular strain, it was excluded it from the analysis. I went back to generating the network and repeated the steps to generate the network and exported to Excel without that gene. I recorded this in my electronic lab notebook.
- The "production_rates" and "degradation_rates" sheets should had values for each gene.
- The "threshold_b" sheet had a value of 0 for each gene.
- In the "optimization_parameters" sheet, I changed the "alpha" value to 0.02 instead of 0.002.
- I inserted a new worksheet and name it "network_weights".
- I Copied the entire content of the "network" sheet into the "network_weights" sheet.
- I saved and uploaded the Excel Workbook to the wiki and linked to it on the individual journal page.
Data and Files
Conclusion
This week's analysis supported that the profile under study (profile 9) not only had several genes with significant results in regulation during cold shock experiments. This was seen in a variety of gene ontology processes. The results from the GRN network also demonstrated several of the transcription factors that were significantly affected by this cold shock experiment.
Acknowledgements
I consulted my homework partner, Katie Miller, during class whenever I had a question. We did not communicate outside of class. I also consulted a few other classmates during class regarding this assignment. I also asked my professor several questions over the course of completing this assignment. Except for what is noted above, this individual journal entry was completed by me and not copied from another source. Msymond1 (talk) 23:54, 3 April 2024 (PDT)
References
- Dahlquist, K. D., Fitzpatrick, B. G., Camacho, E. T., Entzminger, S. D., & Wanner, N. C. (2015). Parameter estimation for gene regulatory networks from microarray data: cold shock response in Saccharomyces cerevisiae. Bulletin of mathematical biology, 77(8), 1457-1492. DOI: 10.1007/s11538-015-0092-6
- Ernst, J., & Bar-Joseph, Z. (2006). STEM: a tool for the analysis of short time series gene expression data. BMC bioinformatics, 7(1), 191. DOI: 10.1093/bioinformatics/bti1022
- Gene Ontology Resource. (n.d.). Gene Ontology Resource. Retrieved April 3, 2024, from http://geneontology.org/
- LMU BioDB 2024. (2024). Week 10. Retrieved April 2, 2024, from https://xmlpipedb.cs.lmu.edu/biodb/spring2024/index.php/Week_10
- Teixeira, M. C., Viana, R., Palma, M., Oliveira, J., Galocha, M., Mota, M. N., ... & Monteiro, P. T. (2023). YEASTRACT+: a portal for the exploitation of global transcription regulation and metabolic model data in yeast biotechnology and pathogenesis. Nucleic Acids Research, 51(D1), D785-D791. DOI: 10.1093/nar/gkac1041
User Page
Assignment Pages
Individual Journal Pages
- MSymond1 Week 1
- MSymond1 Week 2
- MSymond1 KMill104 Week 3
- NeMO_Week4
- MSymond1 Week 5
- MSymond1 Week 6
- MSymond1 Week 8
- MSymond1 Week 9
- MSymond1 Week 10
- MSymond1 Week 12
- MSymond1 Week 13
- MSymond1 Week 15