Hivanson Week 5
Purpose
The purpose of the week 5 individual journal assignment was to familiarize us with microarrays, their purpose, and how to analyze their results. We were provided with different ways that microarrays could be used, and the Campbell textbook and questions were provided in order to deepen our understanding of microarrays through critical thinking and knowledge application about DNA microarrays.
Questions
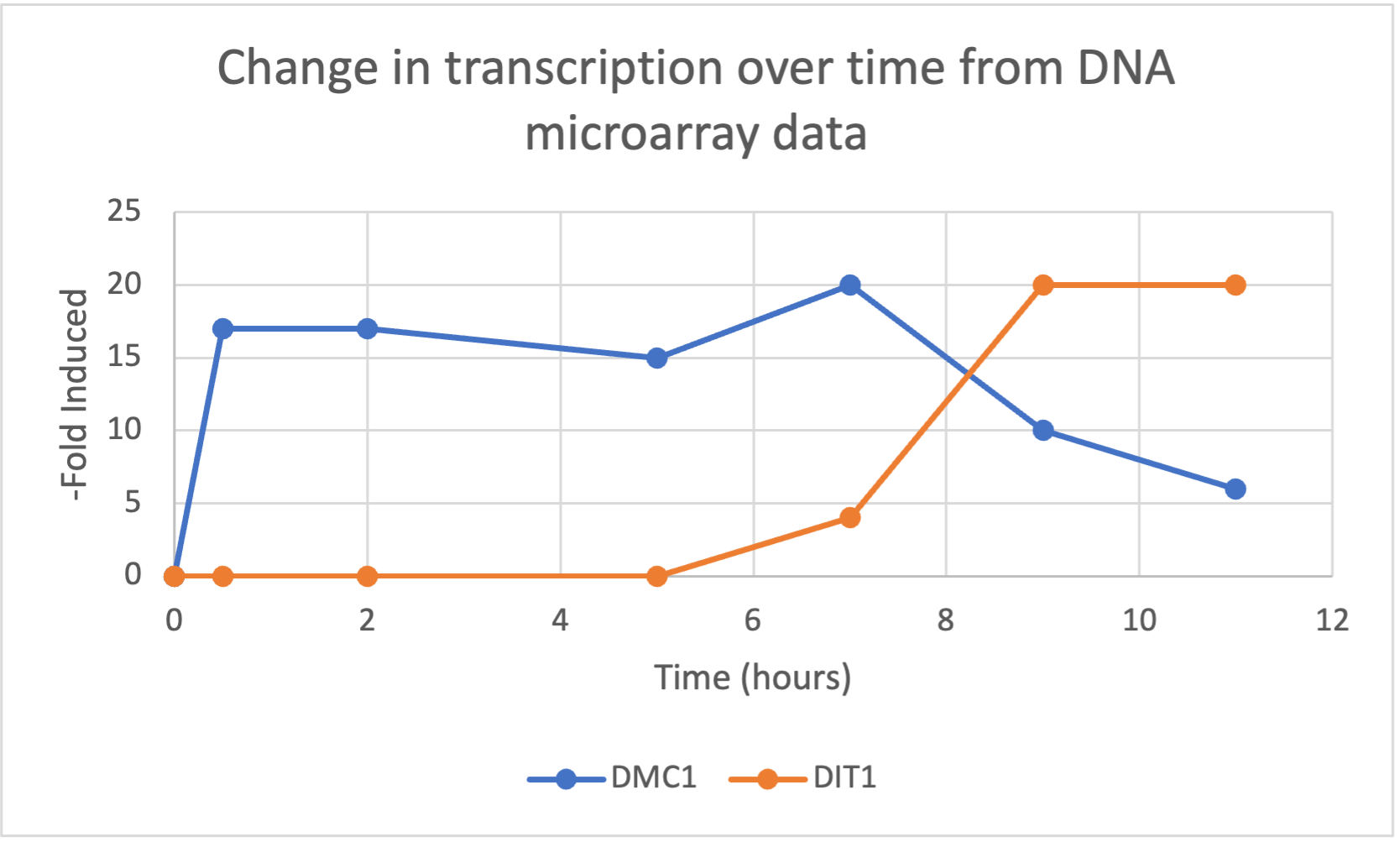
- (Question 5, p. 110) Choose two genes from Figure 4.6b (PDF of figures on Brightspace) and draw a graph to represent the change in transcription over time. You can either create your plot in Excel and put the image up on your wiki page or you can do it by hand and upload a picture or scan.
- (Question 6b, p. 110) Look at Figure 4.7, which depicts the loss of oxygen over time and the transcriptional response of three genes. These data are the ratios of transcription for genes X, Y, and Z during the depletion of oxygen. Using the color scale from Figure 4.6, determine the color for each ratio in Figure 4.7b. (Use the nomenclature "bright green", "medium green", "dim green", "black", "dim red", "medium red", or "bright red" for your answers.)
- (Question 7, p. 110) Were any of the genes in Figure 4.7b transcribed similarly? If so, which ones were transcribed similarly to which ones?
At hours 1 and 3, all genes (X, Y, Z) are similar, with all black at hour 1 and all dim to medium shades of red at hour 3. At hour 5, genes X and Y are similar (black and dim green, with the dim green being dimmer than the dim red of gene Z at this point). At hour 9 X and Y are similar, both having shades of green. Genes X and Y appear to be transcribed the most similarly based on their oxygen gas consumption over time.
- (Question 9, p. 118) Why would most spots be yellow at the first time point? I.e., what is the technical reason that spots show up as yellow - where does the yellow color come from? And, what would be the biological reason that the experiment resulted in most spots being yellow?
Most spots show yellow at the first timepoint with a ~1:1 ratio of green and red. The technical reason for most spots showing yellow at the first time point is due to the mixing of red and green light to make yellow light. The biological reason behind this is that there is not yet a change in a gene’s transcription at the 0 time point, and a ratio of 1:1 is treated as the baseline. control and experimental growth conditions
- (Question 10, p. 118) Go to the Saccharomyces Genome Database and search for the gene TEF4; you will see it is involved in translation. Look at the time point labeled OD 3.7 in Figure 4.12, and find the TEF4 spot. Over the course of this experiment, was TEF4 induced or repressed? Hypothesize why TEF4’s change in expression was part of the cell’s response to a reduction in available glucose (i.e., the only available food).
TEF4 appears a light green color, indicating that it was repressed over the course of the experiment. TEF4's decrease in expression when glucose availability was reduced could be because the yeast cell may be unable to undergo translation when food is reduced and would therefore have no need for TEF4 induction.
- (Question, 11, p. 120) Why would TCA cycle genes be induced if the glucose supply is running out?
TCA cycle genes should be induced when glucose is scarce in order to continue to produce ATP via the TCA cycle, which does not require glucose, rather than via glycolysis, which requires glucose.
- (Question 12, p. 120) What mechanism could the genome use to ensure genes for enzymes in a common pathway are induced or repressed simultaneously?
The genome could use an operon system; a group of genes, typically involved in a common pathway, that are transcribed (or not transcribed) simultaneously.
- (Question 13, p. 121) Consider a microarray experiment where cells deleted for the repressor TUP1 were subjected to the same experiment of a timecourse of glucose depletion where cells at t0 (plenty of glucose available) are labeled green and cells at later timepoints (glucose depleted) are labeled red. What color would you expect the spots that represented glucose-repressed genes to be in the later time points of this experiment?
Glucose-repressed genes will not be repressed in the absence of repressor TUP1. These glucose-repressed genes will turn red throughout the experiment, as they will be transcribed the same in glucose-depletion conditions as in glucose-rich conditions (transcription will continue), marking the spots for these genes as red as transcription is induced at later time points of the experiment.
- (Question 14, p. 121) Consider a microarray experiment where cells that overexpress the transcription factor Yap1p were subjected to the same experiment of a timecourse of glucose depletion where cells at t0 (plenty of glucose available) are labeled green and cells at later timepoints (glucose depleted) are labeled red. What color would you expect the spots that represented Yap1p target genes to be in the later time points of this experiment?
Yap1p enables resistance to environmental distress. Glucose depletion could be a form of distress for a yeast cell and therefore would trigger transcription of Yap1p target genes. The induced transcription of these target genes would lead to the spots showing as red in later time points of the experiment.
- (Question 16, p. 121) Using the microarray data, how could you verify that you had truly deleted TUP1 or overexpressed YAP1 in the experiments described in the above questions?
Deletion of TUP1 and overexpression of Yap1 would both lead to red spots at later time points. TUP1 should immediately begin transcription, turning red sooner in the experiment and staying red throughout the glucose depletion, theoretically not increasing or decreasing. Yap1 overexpression should show up in later time spots, as the Yap1 will not transcribe until the yeast cell begins to respond to low glucose as an environmental stressor; Yap1 will turn red at later time points, but stay black or even green at earlier time points.
Acknowledgments
I worked with my homework partner, Katie Miller to complete this assignment. Katie and I communicated via text message on 2/14/2024, and she helped me understand the logic behind answering Question 6b in this assignment.
Except for what is noted above, this individual journal entry was completed by me and not copied from another source
Hivanson (talk) 22:54, 14 February 2024 (PST)
References
- Alberts et al. (2002) Molecular Biology of the Cell, Ch. 8: Microarrays
- Brown, P.O. & Botstein, D. (1999) Exploring the new world of the genome with DNA microarrays Nature Genetics 21: 33-37. https://doi.org/10.1038/4462
- Campbell, A.M. and Heyer, L.J. (2003), “Chapter 4: Basic Research with DNA Microarrays”, in Discovering Genomics, Proteomics, and Bioinformatics, Cold Spring Harbor Laboratory Press, pp. 107-124. (Available on Brightspace)
- CBS News. (2012, February 12). Deception at Duke. https://www.youtube.com/watch?v=eV9dcAGaVU8
- DataOne. (n.d.). Tutorials on Data Management | Lesson 2: Data Sharing.
- DeRisi, J.L., Iyer, V.R., and Brown, P.O. (1997) Exploring the Metabolic and Genetic Control of Gene Expression on a Genomic Scale. Science 278: 680-686. DOI: https://doi.org/10.1126/science.278.5338.680
- 11.7: Gene Regulation - Operon Theory. (2016, July 10). Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_(OpenStax)/11%3A_Mechanisms_of_Microbial_Genetics/11.07%3A_Gene_Regulation_-_Operon_Theory
- LMU BioDB 2024. (2024). Week 5. Retrieved February 14, 2024, from https://xmlpipedb.cs.lmu.edu/biodb/spring2024/index.php/Week_5
- Saccharomyces Genome Database. (n.d.) TEF4 / YKL081W. Retrieved February 14, 2024, from https://www.yeastgenome.org/locus/S000001564
- Hivanson
- Hivanson Week 1 | Week 1 Assignment
- Hivanson Week 2 | Week 2 Assignment
- IMD3 Hivanson and Nstojan1 Week 3 | Week 3 Assignment
- NeMO Week 4 | Week 4 Assignment
- Hivanson Week 5 | Week 5 Assignment
- Hivanson Week 6 | Week 6 Assignment
- Hivanson Week 8 | Week 8 Assignment
- Hivanson Week 9 | Week 9 Assignment
- Hivanson Week 10 | Week 10 Assignment
- Hivanson Week 12 | Week 12 Assignment
- Hivanson Week 13 | Week 13 Assignment
- Hivanson Week 14 | Week 14 Assignment
- Hivanson Week 15 | Week 15 Assignment
- Main page